Preclinical Imaging
 Preclinical imaging is the visualization of living animals for research purposes, such as drug development. Imaging modalities have long been crucial to the researcher in observing changes, either at the organ, tissue, cell, or molecular level, in animals responding to physiological or environmental changes. Imaging modalities that are non-invasive and in vivo have become especially important to study animal models longitudinally.
Preclinical imaging is the visualization of living animals for research purposes, such as drug development. Imaging modalities have long been crucial to the researcher in observing changes, either at the organ, tissue, cell, or molecular level, in animals responding to physiological or environmental changes. Imaging modalities that are non-invasive and in vivo have become especially important to study animal models longitudinally.
Translational research is changing the practice of modern medicine and the way in which health problems are approached and solved. The use of small-animal models in basic and preclinical sciences is a major keystone for these kinds of research and development strategies, representing a bridge between discoveries at the molecular level and clinical implementation in diagnostics and/or therapeutics.
The most suitable modalities for small-animal in vivo imaging applications are based on nuclear medicine techniques (essentially, positron emission tomography [PET] and single photon emission computed tomography [SPECT]), optical imaging (OI), computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy imaging (MRSI), and ultrasound. Each modality has intrinsic advantages and limitations. More recently, aiming to overcome the inherent limitations of each imaging modality, multimodality devices designed to provide complementary information upon the pathophysiological process under study have gained popularity. The combination of high-resolution modalities, like micro-CT or micro-MRI, with highly sensitive techniques providing functional information, such as micro-PET or micro-SPECT, will continue to broaden the horizons of research in such key areas as infection, oncology, cardiology, and neurology, contributing not only to the understanding of the underlying mechanisms of disease, but also providing efficient and unique tools for evaluating new chemical entities and candidate drugs. The added value of small-animal imaging techniques has driven their increasing use by pharmaceutical companies, contract research organizations, and research institutions.
Modalities Available
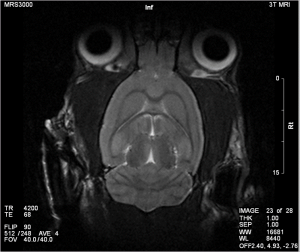
Magnetic Resonance Imaging (MRI)
MRI exploits the nuclear magnetic alignments of different atoms inside a magnetic field to generate images. MRI machines consist of large magnets that generate magnetic fields around the target of analysis. These magnetic fields cause paramagnetic atoms such as hydrogen, gadolinium, and manganese to align themselves in a magnetic dipole along the magnetic fields, created by the radiofrequency (RF) coils inside the MRI machine. What the machine captures from the subject is the relaxation of the atoms as they return to their normal alignment when the RF pulse is temporarily ceased. With this data, a computer will generate an image of the subject based on the resonance characteristics of different tissue types.
Advantages
The advantages of the micro-MRI is that it has good spatial resolution, up to 100 µm and even 25 µm in very high strength magnetic fields. It also has excellent contrast resolution to distinguish between normal and pathological tissue. MRI can be used in a wide variety of applications, including anatomical, functional, and molecular imaging. Furthermore, since MRI’s mechanism is based on a magnetic field, it is much safer compared to radiation based imaging modalities such as CT and PET.
Computed Tomography (CT)
CT imaging works through X-rays that are emitted from a focused radiation source that is rotated around the test subject placed in the middle of the CT scanner. The X-ray is attenuated at different rates depending on the density of tissue it is passing through, and is then picked up by sensors on the opposite end of the CT scanner from the emission source.
In contrast to traditional 2D X-ray, since the emission source in a CT scanner is rotated around the animal, a series of 2D images can then be combined into 3D structures by the computer.
Single Photon Emission Computed Tomography (SPECT)
SPECT also images living systems through γ-rays emitted from within the subject. Unlike PET, the radioisotopes used in SPECT (such as technetium-99m) emit γ-rays directly, instead of from annihilation events of a positron and electron. These rays are then captured by a γ-camera rotated around the subject and subsequently rendered into images.
Positron Emission Tomography (PET)
PET images living systems by recording high-energy γ-rays emitted from within the subject. The source of the radiation comes from positron-emitting-bound biological molecules, such as 18F-FDG (fludeoxyglucose), which is injected into the test subject. As the radioisotopes decay, they emit positrons which annihilates with electrons found naturally in the body. This produces 2 γ-rays at ~180° apart, which are picked up by sensors on opposite ends of the PET machine. This allows individual emission events to be localized within the body, and the data set is reconstructed to produce images.
Optical Imaging
Optical Imaging is divided into fluorescence and bioluminescence.
Fluorescence Imaging
Fluorescence imaging works on the basis of fluorochromes inside the subject that are excited by an external light source, and which emit light of a different wavelength in response. Traditional fluorochromes include GFP, RFP, and their many mutants. However significant challenges emerge in vivo due to the autofluorescence of tissue at wavelengths below 700 nm. This has led to a transition to near-infrared dyes and infrared fluorescent proteins (700 nm-800 nm) which have demonstrated much more feasibility for in vivo imaging due to the much lower autofluorescence of tissue and deeper tissue penetration at these wavelengths.
Bioluminescence Imaging
Bioluminescence imaging, on the other hand, is based on light generated by chemiluminescent enzymatic reactions. In both fluorescence and bioluminescence imaging, the light signals are captured by Charged Coupled Device (CCD) cameras cooled up to -150 °C, making them extremely light-sensitive. In events where more light is produced, less sensitive cameras or even the naked eye can be used to visualize the image.
References:
http://link.springer.com/article/10.1007%2Fs40291-013-0062-3
http://en.wikipedia.org/wiki/Preclinical_imaging
